- Home Page
- About Surya
-
Business Segment
- Intermediates
- Benzohydrol | C13H12O
- Meta Hydroxy Acetophenone
- Meta Bromo Anisole
- Meta Bromo Phenol
- Meta Amino Benzoic Acid
- Cis Bromo Benzoate
- 4-(Cyclopropylcarbonyl)-alpha,alpha-dimethylbenzeneacetic Acid
- 2-(4-(Cyclopropanecarbonyl)Phenyl)-2-Methylpropanoic Acid
- Methyl 2-(4-(4-Chlorobutanoyl)Phenyl)-2-Methylpropanoate
- 4- Hydroxycoumarin
- 2,3-Lutidine C7H9N
- Meta Bromo Benzaldehyde
- Meta Phenoxy Benzaldehyde
- Tetra Butyl Ammonium Bromide
- 3-Bromo-N,N- Dimethylaniline
- Benzhy drol
- 4- Hydroxycoumarin
- 2.,3 Lutidine
- 4- Bromobenzaldehyde
- 4-Bromobenzyl Alchol
- 2-(4-(4-(4-(Hydroxy Diphenyl Methyl)-1- Piper-Idine)-1- Oxobutyl)Phenyl)-2,2- Dimethyl Acetic Acid Methyl Ester
- Methyl 2-(4-(4-Chlorobutanoyl)Phenyl)-2- Methyl Propanoate
- 4-(Cyclopropyl-Oxo-Methyl)-Alpha, Alpha- Dimethyl Phenyl Acetic Acid
- 4-(Cyclopropyl-Oxo-Methyl)-Alpha, Alpha- Dimethyl Phenyl Acetic Acid Cyclohexylamine Salt
- Alpha, Alpha Dimethyl Phenyl Acetic Acid
- Isonipecotic Acid
- Meta Bromo Benzyldehyde
- Meta Phenoxy Benzyl Alcohol
- Meta Bromo Nitro Benzene
- 4 Hydroxycoumarin
- Mannich HCL
- Para Bromobenzaldehyde
- Para Bromobenzyl Alcohol
- Para Bromo Toulene
- Para Bromo Aniline
- Acetyl Hydrazide
- 2-Amino -3, 5- Dibromo Benzaldehyde
- 3-Hydroxy-A-(N-Methyl-N-Benzylamino) Acetophenone HCL
- Methyl Isonipecotate
- Ethyl Isonipecotate
- Tetrabutylammonium Bromide
- 3-(1-Cyanoethyl) Benzoic Acid (CEBA)
- Ethyl Benzoate
- 2-Amino-5-Methyl Thiazole
- 5-Bromo-2-Chlorobenzoic Acid
- APIs
- Agro Chemicals
- Perfumery & Fragrance
- Intermediate List
- 2,3-Diamino Pyridine
- 5-Bromo-2-Chlorobenzoic Acid
- Ethyl Benzoate
- 3-(1-Cyanoethyl) Benzoic Acid (Ceba)
- Trifluoroacetic Acid Isopropyl Ester
- Tetrabutylammonium Bromide
- Ethyl Isonipecotate
- Methyl Isonipecotate
- 3-Hydroxy-(N-Methyl-N Benzylamino) Acetophenone HCL
- Diphenyl(Pipe-ridin-4-YL) Methanol (Azacyclonol)
- 2-Amino-3, 5- Dibromo Benzaldehyde
- Acetyl Hydrazide
- Para Bromo Aniline
- Para Bromo Toluene
- Para Bromobenzyl Alcohol
- Para Bromobenzaldehyde
- Para Amino Benzonitrile
- Meta Bromo Nitro Benzene
- Meta Phenoxy Benzyl Alcohol
- Meta Bromo Benzaldehyde
- Meta Amino Benzoic Acid
- Meta Anisidine
- Meta Bromo Phenol
- Meta Bromo Anisole
- 6-Bromo-2Methoxy-3 Benzyl Quinoline
- Isonipecotic Acid
- Alpha Alpha Dimethyl Phenyl Acetic Acid
- 4-(Cyclopropyl-Oxo-Methyl)-Alpha, Alpha Dimethyl Phenyl Acetic Acid
- Methyl 2-(4-(Chlorobutanoyl)Phenyl)-2-Methylpropanoate
- APIS List
- API List
- Active Pharmaceutical Ingredients
- Fexofenadine IP/BP/USP
- Ambroxol IP/BP/USP
- Bromhexine Hcl
- Ketoprofen IP/BP/USP
- Lidocaine IP
- Quetiapine Fumarate
- Verapamil HCL
- Metoprolol Succinate
- Meloxicam (C14H13N3O4S2)
- Sitagliptin Phosphate
- Bromhexine HCL
- Tamsulosin HCL
- Meta Anisidine
- Etoricoxib CAS 202409-33-4
- Metoprolol Tartarate (BP)
- Parecoxib Sodium
- 3-Bromoaniline C6H6BrN
- Fexofenadine HCL
- Ketoconazole (IP, BP, USP 30)
- Amlodipine Besylate (BP)
- Cis -Tosylate
- Meta Bromoaniline
- p- Fluoromethoxybenzene
- 2-[4-[4-[4-(Hydroxydiphenylmethyl)-1-Piperidinyl]-1-Oxobutyl]Phenyl]-2,2-Dimethylacetic Acid Methyl Ester
- 4- Fluoroanisole
- Bromhexine HCL
- Azacyclonol Pharmacutical Intermediate
- Fluconazole Powder
- Meta Bromo Aniline
- Meta Bromoaniline
- 2-Amino Pyridine
- 3-(Trifluoromethyl)-5,6,7,8-Tetrahydro-[1,2,4]Triazolo[4,3-A] Pyrazine Hydrochloride
- 2,3-Diamino Pyridine
- Intermediates
- Research & Development
- We Care
- Contact Us
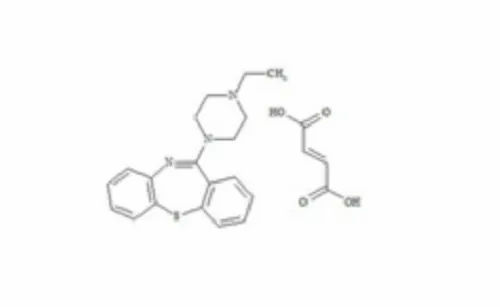
Quetiapine Fumarate
8500 INR/Kilograms
Product Details:
- Molecular Weight 505.6 g/mol
- Taste Bitter
- Solubility Soluble in Dimethyl Formamide
- Shelf Life 3 years
- Molecular Formula C21H25N3O2SC4H4O4
- HS Code 29349900
- Heavy Metal (%) NMT 0.001%
- Click to View more
X
Quetiapine Fumarate Price And Quantity
- 8500 INR/Kilograms
- 100 Kilograms
- Tablet
- 98.0% to 102.0% (on anhydrous basis)
- USP/IP/EP
- Positive IR, HPLC and UV spectrum
- 25 Kg
- NMT 0.1%
- Atypical antipsychotic used in treatment of schizophrenia and bipolar disorder
- Complies as per USP/ICH
- Double polyethylene bags placed in HDPE drum
- 500 Kg/Month
Quetiapine Fumarate Product Specifications
- Quetiapine Fumarate
- 173177C
- 111974-72-2
- NO
- 0.5% max
- 99%
- Available on request
- 505.6 g/mol
- White to Off-White Powder
- Bitter
- Soluble in Dimethyl Formamide
- 3 years
- Quetiapine Fumarate
- C21H25N3O2SC4H4O4
- Pharmaceutical
- 29349900
- Powder
- NMT 0.001%
- Tablet
- 98.0% to 102.0% (on anhydrous basis)
- USP/IP/EP
- Positive IR, HPLC and UV spectrum
- 25 Kg
- NMT 0.1%
- Atypical antipsychotic used in treatment of schizophrenia and bipolar disorder
- Complies as per USP/ICH
- Double polyethylene bags placed in HDPE drum
- 500 Kg/Month
Quetiapine Fumarate Trade Information
- 1000 Kilograms Per Day
- 1 Week
- Contact us for information regarding our sample policy
Product Description
Quetiapine Fumarate Description :
Chemical Name : Quetiapine fumarate is the fumarate salt of 224dibenzobf14thiazepin11yl1piperazinylethoxyethanol
Molecular Formula : C42H50N6O4S2 C4H4O4
Molecular Weight : 88311 gmol fumarate salt
Appearance: Quetiapine fumarate appears as a white to offwhite crystalline powder
Assay Contains not less than 980 and not more than 1020 of C42H50N6O4S2 C4H4O4 calculated on the dried basis
Identification : Identification of quetiapine fumarate is confirmed using infrared absorption spectrophotometry and chromatographic methods
Solubility : Quetiapine fumarate is practically insoluble in water freely soluble in methanol sparingly soluble in ethanol and slightly soluble in dichloromethane
Melting Point : The melting point of quetiapine fumarate is between 181C and 183C
Storage : Store quetiapine fumarate in a wellclosed container protected from light and moisture
Usage : Quetiapine fumarate is an atypical antipsychotic used in the treatment of schizophrenia bipolar disorder and major depressive disorder It acts by antagonizing multiple neurotransmitter receptors including serotonin 5HT2A and 5HT2C dopamine D1 and D2 histamine H1 and adrenergic alpha1 and alpha2 receptors
This comprehensive description ensures that quetiapine fumarate meets the stringent quality standards required for pharmaceutical use
Consistent Quality Meets Global Standards
Our Quetiapine Fumarate strictly complies with USP/IP/EP standards, ensuring reliability and safety for pharmaceutical applications. Every batch is thoroughly analyzed for chemical integrity, purity, and residual solvents, meeting the requirements for international regulatory bodies and industry needs.
Secure & Hygienic Packaging
Packaged in double polyethylene bags placed inside HDPE drums, this product is designed to maintain its stability, prevent contamination, and provide robust protection during transportation or storage. This thoughtful packaging supports both bulk handling and convenient usage for tablet formulation manufacturing.
FAQ's of Quetiapine Fumarate:
Q: How is Quetiapine Fumarate typically used in pharmaceutical formulations?
A: Quetiapine Fumarate is commonly used in tablet formulations as an active pharmaceutical ingredient for the treatment of psychiatric disorders like schizophrenia and bipolar disorder.Q: What are the quality specifications for Quetiapine Fumarate?
A: This product meets USP/IP/EP standards, is positively identified by IR, HPLC, and UV spectrum, and contains 98.0%-102.0% assay (anhydrous basis). Related substances are kept below 0.1%, and heavy metals below 0.001%.Q: When should Quetiapine Fumarate be ordered and how is it supplied?
A: Quetiapine Fumarate can be ordered year-round with a minimum order quantity of 25 kg. It is supplied in secure double polyethylene bags inside HDPE drums to maintain quality during storage and shipment.Q: Where is Quetiapine Fumarate manufactured and distributed from?
A: It is manufactured, supplied, and exported from India by authorized distributors, exporters, and manufacturers with a monthly production capacity of 500 kg.Q: What is the process for quality assurance of this ingredient?
A: Each batch undergoes rigorous identification using IR, HPLC, and UV spectrum, as well as checks for related substances, residual solvents, heavy metals, and assay to ensure compliance with global pharmacopeial standards.Q: What are the benefits of using this particular Quetiapine Fumarate?
A: Clients benefit from high purity (99%), global pharmacopeial compliance, secure packaging, reliable supply, and detailed quality assurances, making it an excellent choice for pharmaceutical manufacturing purposes.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email






 Send Inquiry
Send Inquiry Send SMS
Send SMS
